Kwanan nan, Shanghai Chuangkun Biotechnology Co., Ltd. ya sami takardar shaidar rajista ta biyu na Indonesia FDA don TB/NTM Nucleic Acid detection kit (lyophilized), wanda aka samu ta hanyar (12+3) nau'in gano PCR na HPV wata daya da ya wuce.wanda ya bayyana cewa kayayyakin Chuangkun Biotech sun sami izini daga Indonesia FDA, da inganta kayayyakin da suka ɓullo da kansu, da kuma ba da tallafi mai ƙarfi ga Chuangkun Biotech don ƙara faɗaɗa kasuwannin duniya.
A cewar rahoton Hukumar Lafiya ta Duniya ta Duniya na 2022, kimanin mutane miliyan 10.6 za su kamu da tarin fuka a shekarar 2021. Yawan karuwar da kashi 4.5% ya zarce na shekarar 2020. Yayin da mutane miliyan 1.6 suka mutu sakamakon tarin fuka a bara (ciki har da 6.7% na daga cikin masu dauke da kwayar cutar HIV).A geographically, yawancin cututtukan tarin fuka a cikin 2021 sun kasance a cikin yankunan WHO na Kudu maso Gabas Asia (45%), Afirka (23%) da Yammacin Pacific (18%), tare da ƙananan hannun jari a Gabashin Bahar Rum (8.1%), Amurka. (2.9%) da Turai (2.2%).Yin la'akari da Cutar ta COVID-19, rigakafin cutar tarin fuka da sarrafawa yana ƙara yin tsauri ga Shirin Kawo Cutar Tarin Fuka na WHO.
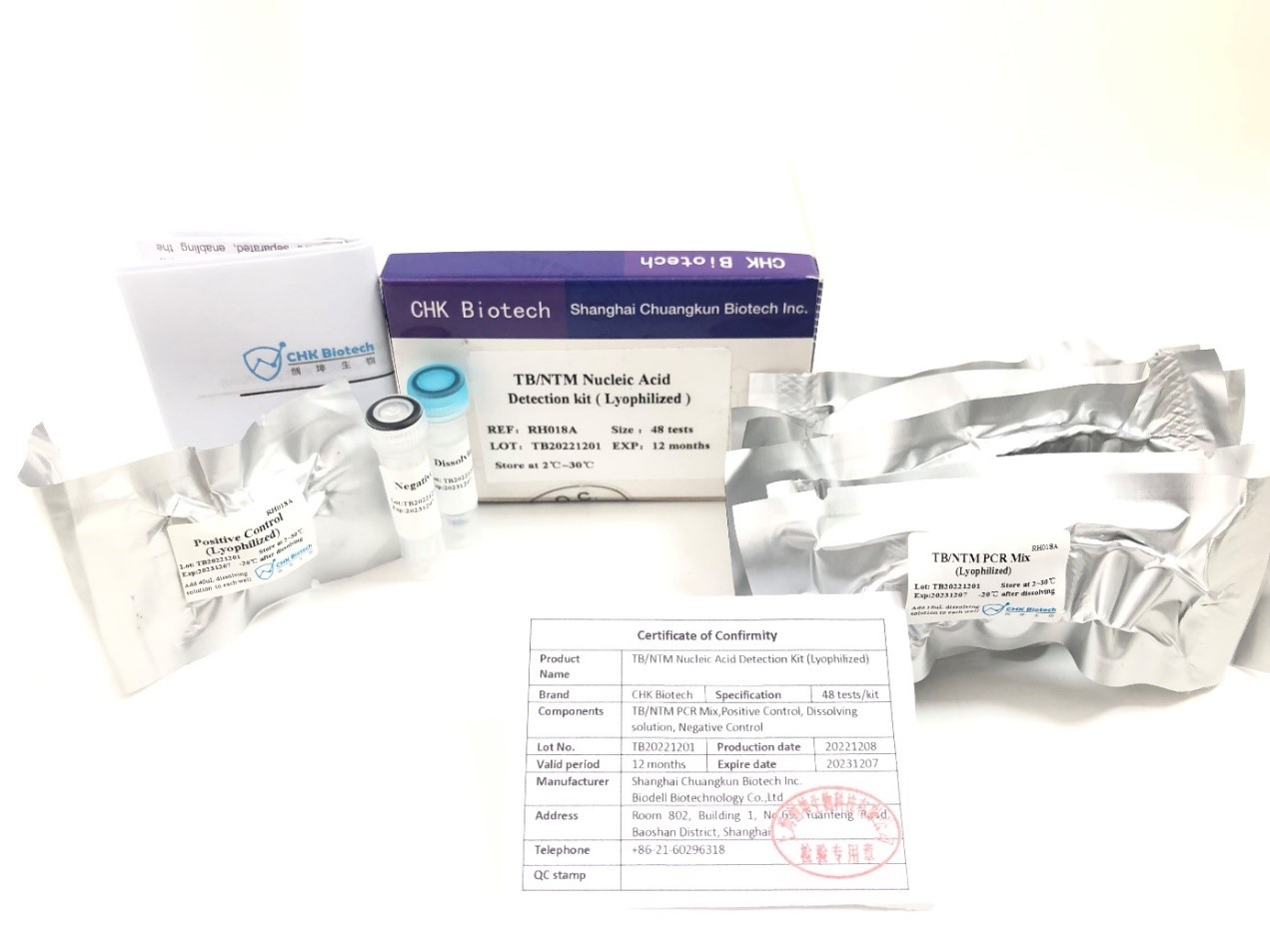
TB / NTM Nucleic acid detection kit daga CHUANGKUN Biotech an halicce shi ta hanyar hanyar lyophilization .Tsarin zai magance rashin lafiyar sufuri na dogon lokaci don kayan aikin PCR na al'ada, kuma yana kawo kyakkyawan daidaito da sakamako mai mahimmanci fiye da jin dadi don amfaninmu.
Shanghai Chuangkun Biotechnology Co., Ltd. ya sami takardar shaidar rajista ta biyu na Indonesia FDA don TB/NTM Nucleic Acid gano kayan, yana nuna amincewa da FDA ta Indonesia.Chuangkun Biotech za ta dukufa wajen fakewa a cikin rigakafin cutar tarin fuka da kuma sarrafa shi sosai.
Lokacin aikawa: Juni-08-2023

 中文
中文